2.
DISSOCIATION OF NH-ACIDS
2.1. Conjugated NH-acids (N+H-acids)
2.1.1. Conjugated NH-acids
with noncyclic N
2.1.1.1. Conjugated acids of amines
2.1.1.1.1. Aliphatic amines
2.1.1.1.1.1. Alkylamines
2.1.1.1.1.1.1. Primary amines RN+H3
2.1.1.1.1.1.2. Secondary amines R1R2N+H2
2.1.1.1.1.1.3. Tertiary amines R1R2R3N+H
2.1.1.1.1.1.3.1. R1=R2=R3 = alkyl
2.1.1.1.1.1.3.2. R1,R2,R3 = alkyl
2.1.1.1.1.2. Alkenylamines, alkynylamines
2.1.1.1.1.3. Substituted alkylamines
2.1.1.1.1.3.1. Alkyl substituted by functional
group
2.1.1.1.1.3.1.1. Alkyl substituted by halogen
2.1.1.1.1.3.1.1.1.
Primary amines RN+H3
2.1.1.1.1.3.1.1.2. Secondary amines R1R2N+H2
2.1.1.1.1.3.1.1.3.
Tertiary amines R1R2R3N+H
2.1.1.1.1.3.1.2. Alkyl substituted by OH-group
2.1.1.1.1.3.1.2.1.
Primary alkanolamines RN+H3
2.1.1.1.1.3.1.2.2. Secondary alkanolamines R1R2N+H2
2.1.1.1.1.3.1.2.3.
Tertiary alkanolamines R1R2R3N+H
2.1.1.1.1.3.1.3. Alkyl substituted by OX-group
2.1.1.1.1.3.1.4. Alkyl substituted by OOCX-group
2.1.1.1.1.3.1.5. Alkyl substituted by COX-group
2.1.1.1.1.3.1.6. Alkyl substituted by COOX-group
2.1.1.1.1.3.1.7. Alkyl substituted by CONX2-group
2.1.1.1.1.3.1.8. Alkyl
substituted by CSNX2-group
2.1.1.1.1.3.1.9. Alkyl substituted by NHCOX-group
2.1.1.1.1.3.1.10. Alkyl substituted by NHCOCH=NOH-group
2.1.1.1.1.3.1.11. Alkyl substituted by =NOH-group
2.1.1.1.1.3.1.12. Alkyl substituted by SR-group
2.1.1.1.1.3.1.13. Alkyl substituted by SC(O)X-group
2.1.1.1.1.3.1.14. Alkyl substituted by CN-group
2.1.1.1.1.3.1.15. Alkyl substituted by P(O)X2-group
2.1.1.1.1.3.1.16. Alkyl substituted by P(O)(OX2)-group
2.1.1.1.1.3.1.17. Alkyl substituted by OP(O)(OX2)-group
2.1.1.1.1.3.1.18. Alkyl substituted by SiX3-group
2.1.1.1.1.1.1. Primary amines RN+H3
2.1.1.1.1.1.2. Secondary amines R1R2N+H2
2.1.1.1.1.1.3. Tertiary amines R1R2R3N+H
2.1.1.1.1.3.1.19. Alkyl substituted by NO2-group
2.1.1.1.1.3.1.20. Alkyl substituted by NHNH2-group
2.1.1.1.1.3.1.21. Alkyl substituted by SO3--group
2.1.1.1.1.3.1.22. Alkyl substituted by SnX3-group
2.1.1.1.1.3.1.23. Alkyl substituted by GeX3-group
2.1.1.1.1.3.1.24. Alkyl substituted by SeCOX-group
2.1.1.1.1.3.2. Alkyl substituted by
carbocycle
2.1.1.1.1.3.2.1. Alkyl substituted by phenyl and substituted phenyl
2.1.1.1.1.3.2.1.1. Benzylamines
2.1.1.1.1.3.2.1.1.1. Primary
benzylamines PhCH2N+H3
2.1.1.1.1.3.2.1.1.2. Secondary amines
PhCH2N+H2R1
2.1.1.1.1.3.2.1.1.3.
Tertiary amines PhCH2N+HR1R2
2.1.1.1.1.3.2.1.2. Other by phenyl
substituted alkylamines
2.1.1.1.1.3.2.1.2.1.
Primary amines RN+H3
2.1.1.1.1.3.2.1.2.2. Secondary amines R1R2N+H2
2.1.1.1.1.3.2.1.2.3.
Tertiary amines R1R2R3N+H
2.1.1.1.1.3.2.2. Alkyl substituted by other carbocycle
2.1.1.1.1.3.3. Alkyl substituted by
heterocycle
2.1.1.1.1.3.3.1. Alkyl substituted by monocyclic heterocycle
2.1.1.1.1.3.3.1.1. Primary amines RN+H3
2.1.1.1.1.3.3.1.2. Secondary amines R1R2N+H2
2.1.1.1.1.3.3.1.3. Tertiary amines R1R2R3N+H
2.1.1.1.1.3.3.2. Alkyl substituted by polycyclic heterocycle
2.1.1.1.1.3.4. Alkyl substituted by
various group
2.1.1.1.1.3.4.1. Alkyl substituted by phenyl and OH-group
2.1.1.1.1.3.4.2. Alkyl substituted by phenyl and OOCX-group
2.1.1.1.1.3.4.3. Alkyl substituted by phenyl and NHCOX-group
2.1.1.1.1.3.4.4. Alkyl substituted by phenyl and various functional
group
2.1.1.1.1.3.4.5. Alkyl substituted by various functional group
2.1.1.1.1.4. Substituted alkenyl- and alkynylamines
2.1.1.1.1.5. Amines contained various functional groups
2.1.1.1.1.5.1. Amines contained halogen
2.1.1.1.1.5.2. Amines contained
OH-group
2.1.1.1.1.5.3. Amines contained
O(alkyl)-group
2.1.1.1.1.5.4. Amines contained CONX2-group
2.1.1.1.1.5.5. Amines contained CSNX2-group
2.1.1.1.1.5.6. Amines contained
NHCOR-group
2.1.1.1.1.5.7. Amines contained NXSO2X-group
2.1.1.1.1.5.8. Amines contained
NHX-group (X = cycle)
2.1.1.1.1.5.9. Amines contained NXP(O)X2-group
2.1.1.1.1.5.10. Amines contained
NXP(S)X2-group
2.1.1.1.1.5.11. Amines contained N=CX2-group
2.1.1.1.1.5.12. Amines contained
CH=NCOX-group
2.1.1.1.1.5.13. Amines contained CN-group
2.1.1.1.1.5.14. Amines contained P(O)X2-
and P(S)X2-group
2.1.1.1.1.5.15. Amines contained SiX3-group
2.1.1.1.1.5.16. Amines contained SO2R-group
2.1.1.1.1.6. Polyamines
2.1.1.1.1.6.1. Symetric polyamines
2.1.1.1.1.6.1.1. Symetric diamines
2.1.1.1.1.6.1.1.1. Primary diamines
2.1.1.1.1.6.1.1.2. Secondary diamines
2.1.1.1.1.6.1.1.3. Tertiary diamines
2.1.1.1.1.6.1.2. Symetric triamines
2.1.1.1.1.6.1.3. Symetric tetraamines
2.1.1.1.1.6.2. Asymetric polyamines
2.1.1.1.1.6.2.1. Asymetric diamines
2.1.1.1.1.6.2.2. Asymetric triamines
2.1.1.1.2. Cyclic amines
2.1.1.1.2.1. Carbocyclic amines
2.1.1.1.2.1.1. Carbocyclic monoamines
2.1.1.1.2.1.1.1. Saturated carbocyclic amines
2.1.1.1.2.1.1.2. Anilines
2.1.1.1.2.1.1.2.1. Anilines with one substituent RC6H4N+H3
2.1.1.1.2.1.1.2.1.1. R = -H
2.1.1.1.2.1.1.2.1.2. R = alkyl, alkenyl
2.1.1.1.2.1.1.2.1.3. R = functional
group
2.1.1.1.2.1.1.2.1.3.1. R = halogen
2.1.1.1.2.1.1.2.1.3.2. R = -OX
2.1.1.1.2.1.1.2.1.3.3. R = -COX
2.1.1.1.2.1.1.2.1.3.4. R = -NX2, -N+X3
2.1.1.1.2.1.1.2.1.3.5. R = -NHCOX, -NHSO2Ph
2.1.1.1.2.1.1.2.1.3.6. R = -N=NX
2.1.1.1.2.1.1.2.1.3.7. R = -CN
2.1.1.1.2.1.1.2.1.3.8. R = -NO2
2.1.1.1.2.1.1.2.1.3.9. R = -SX
2.1.1.1.2.1.1.2.1.3.10. R = -S(O)X
2.1.1.1.2.1.1.2.1.3.11. R = -SO2X
2.1.1.1.2.1.1.2.1.3.12. R = -SO3-
2.1.1.1.2.1.1.2.1.3.13. R = -PO3H2, -PO3H-
2.1.1.1.2.1.1.2.1.3.14. R = -AsO3H2
2.1.1.1.2.1.1.2.1.3.15. R = -SiX3
2.1.1.1.2.1.1.2.1.3.16. R = -B(OH)2
2.1.1.1.2.1.1.2.1.4. R = substituted
alkyl- or alkenyl
2.1.1.1.2.1.1.2.1.5. R = phenyl or
substituted phenyl
2.1.1.1.2.1.1.2.1.6. R = heterocycle
2.1.1.1.2.1.1.2.2. Anilines with two substituents R1R2C6H3N+H3
2.1.1.1.2.1.1.2.2.1. R1 = R2
= -D
2.1.1.1.2.1.1.2.2.2. R1 = R2
= alkyl
2.1.1.1.2.1.1.2.2.3. R1 =
alkyl, R2 = functional group
2.1.1.1.2.1.1.2.2.4. R1 =
halogen, R2 = functional group
2.1.1.1.2.1.1.2.2.5. R1 = -OH, R2 = functional group
2.1.1.1.2.1.1.2.2.6. R1, R2
= various groups
2.1.1.1.2.1.1.2.3. Anilines with three substituents R1R2R3C6H2N+H3
2.1.1.1.2.1.1.2.4. Anilines with four substituents R1R2R3R4C6HN+H3
2.1.1.1.2.1.1.2.5. Anilines with five substituents R1R2R3R4R5CN+H3
2.1.1.1.2.1.1.3. N-substituted anilines
2.1.1.1.2.1.1.3.1. N-methyl anilines
2.1.1.1.2.1.1.3.2. N-ethyl anilines
2.1.1.1.2.1.1.3.3. N-alkyl anilines
2.1.1.1.2.1.1.3.4. N-phenyl anilines
2.1.1.1.2.1.1.3.5. Other N-substituted
anilines
2.1.1.1.2.1.1.4. N,N-disubstituted anilines
2.1.1.1.2.1.1.4.1. N,N-dimethyl
anilines
2.1.1.1.2.1.1.4.1.1. Nonbridged systems
of N,N-dimethyl anilines
2.1.1.1.2.1.1.4.1.2. Bridged systems of
N,N-dimethyl anilines
2.1.1.1.2.1.1.4.2. N,N-diethyl anilines
2.1.1.1.2.1.1.4.3. N,N-dialkyl anilines
2.1.1.1.2.1.1.4.4. Other
N,N-disubstituted anilines
2.1.1.1.2.1.1.5. Naphthylamines
2.1.1.1.2.1.1.5.1. 1-Naphthylamines
2.1.1.1.2.1.1.5.2. 2-Naphthylamines
2.1.1.1.2.1.1.5.3. N-substituted and
N,N-disubstituted 1-Naphthylamines
2.1.1.1.2.1.1.5.4. N-substituted and
N,N-disubstituted 2-Naphthylamines
2.1.1.1.2.1.1.6. Other carbocyclic amines
2.1.1.1.2.1.2. Carbocyclic polyamines
2.1.1.1.2.1.2.1. Polyaminobenzenes
2.1.1.1.2.1.2.1.1. Symetric polyamines
2.1.1.1.2.1.2.1.1.1. Diaminobenzenes
2.1.1.1.2.1.2.1.1.2.
Diaminoalkylbenzenes
2.1.1.1.2.1.2.1.1.3. Diphenylsystems of
anilines
2.1.1.1.2.1.2.1.1.4. Bridged systems of
anilines
2.1.1.1.2.1.2.1.1.4.1. Diamine
2.1.1.1.2.1.2.1.1.4.2. Tetraamine
2.1.1.1.2.1.2.1.2. Asymetric polyamines
2.1.1.1.2.1.2.1.2.1. Biphenyl systems
of anilines
2.1.1.1.2.1.2.1.2.3. Bridged systems of
anilines
2.1.1.1.2.1.2.2.
Naphthylpolyamines
2.1.1.1.2.1.2.3. Other carbocyclic diamines
2.1.1.1.2.2. Heterocyclic amines
2.1.1.1.2.2.1. Monocyclic amines
2.1.1.1.2.2.1.1. Five-membered heterocyclic amines
2.1.1.1.2.2.1.2. Six-membered heterocyclic amines
2.1.1.1.2.2.2. Polycyclic amines
2.1.1.2.
Conjugated acids of azomethines - R1R2C=N+HR3
2.1.1.2.1. Me2NCH=N+HX
2.1.1.2.2. Me2NCMe=N+HX
2.1.1.2.3. X1X2C=N+HOH
2.1.1.2.4. X1X2C=N+HNX3X4
2.1.1.2.5. (subst. phenyl)CH=N+HX
2.1.1.2.6. (subst. phenyl)CH=N+H(subst.
phenyl)
2.1.1.2.7. (subst. phenyl)CH=N+H(heterocycle)
2.1.1.2.8. (naphthyl)CH=NX
2.1.1.2.9. (heterocycle)CH=N(subst. phenyl)
2.1.1.2.10. (subst. phenyl)CMe=N+H(subst.
phenyl)
2.1.1.2.11. (subst. phenyl)CPh=N+H2
2.1.1.2.12. (subst. phenyl)2C=N+H2
2.1.1.2.13. (subst. phenyl)CPh=N+H(subst.
phenyl)
2.1.1.2.14. (subst. phenyl)CH=CHCH=N+H(subst.
phenyl)
2.1.1.2.15. (subst.
phenyl)CH=CHCH=CHCH=N+H(subst. phenyl)
2.1.1.2.16. Cyclic
azomethines
2.1.1.2.17. Various azomethines
2.1.1.3.
Conjugated acids of azocompounds - [R1N=NR2]H+
2.1.1.3.1. R1, R2 = substituted phenyl
2.1.1.3.2. R1 = substituted phenyl, R2 =
substituted naphthyl
2.1.1.3.3. Various azocompounds
2.1.1.4.
Conjugated acids of azines - [R1CH=NN=CHR2]H+
2.1.1.5.
Conjugated acids of amidines - R1C(=N+HR4)NR2R3
2.1.1.5.1. XC(=N+H2)NH2
2.1.1.5.2. X1C(=N+H2)NHX2
2.1.1.5.3. X1C(=N+H2)NX2X3
2.1.1.5.4. X1C(=N+HX’)NX2X3 (X’ = alkyl)
2.1.1.5.4.1.
XC(=N+HMe)NHMe
2.1.1.5.4.2.
XC(=N+HX’)NMe2
(X’ = alkyl)
2.1.1.5.4.3.
X1C(=N+X’) NX2X3 (X’ = alkyl)
2.1.1.5.4.4. XC(=N+HX’)N(CH2)5 (X’ = alkyl)
2.1.1.5.5. XC(=N+HOH)NH2
2.1.1.5.6. X1C(=N+HNX’2)NX2X3
2.1.1.5.7. X1C[=N+H(subst.
phenyl)]NX2X3
2.1.1.5.7.1. HC[=N+H(subst. phenyl)]NMe2
2.1.1.5.7.2. MeC[=N+H(subst.
phenyl)]NMe(subst. phenyl)
2.1.1.5.7.3. HC(=N+HPh)]NR1R2
2.1.1.5.7.4. MeC[=N+H(subst.
phenyl)]NH(subst. phenyl)
2.1.1.5.7.5. MeC[=N+H(subst.
phenyl)]NMe(subst. phenyl)
2.1.1.5.7.6. X’C[=N+H(subst.
phenyl)]NMe2 (X’ = alkyl)
2.1.1.5.7.7. PhC(=N+H(phenyl)]NH(subst.
phenyl)
2.1.1.5.7.8. PhC[=N+H(subst.
phenyl)]NMe2
2.1.1.5.7.9. PhC[=N+H(subst.
phenyl)]NMe(subst. phenyl)
2.1.1.5.7.10. RC(=N+H(phenyl)]N(CH2)4
2.1.1.5.7.11. RC(=N+H(subst.
phenyl)]N(CH2)5
2.1.1.5.7.12. RC(=N+H(phenyl)]N(CH2)2O
2.1.1.5.8. XC[=N+HCH2(subst.
phenyl)]NMe2
2.1.1.5.9. Various amidines
2.1.1.6.
Conjugated acids of guanidines - R1R2NC(=N+HR5)NR3R4
2.1.1.6.1. RNHC(=N+H2)NH2
2.1.1.6.2. H2NC(=N+HR)NH2
2.1.1.6.3. Me2NC[=N+H(subst.
phenyl)]NMe2
2.1.1.6.4. Various guanidines
2.1.1.7. Conjugated acids of
phosphineimines - R1R2R3P=N+HR4
2.1.1.7.1. (Me2N)3P=N+HR
2.1.1.7.2. Et2PPh=N+H(subst. phenyl)
2.1.1.7.3. (subst. phenyl)PPh2=N+H(subst. phenyl)
2.1.1.7.4. (subst. phenyl)2PPh2=N+H(subst. phenyl)
2.1.1.7.5. (subst. phenyl)3PPh=N+H(subst. phenyl)
![]()
![]()
2.1.1.7.8. Ph3P=N+HN=N(subst. phenyl)
2.1.1.7.9. R1R2R3PPh=N+H(subst. phenyl)
2.1.1.7.10. (subst. phenyl)N=PPh2CH2PPh2=N+H(subst.
phenyl)
2.1.1.7.11. XP+Ph2CH2PPh2=N+H(subst.
phenyl)
2.1.1.7.12. Various
phoshineimines
2.1.2. Conjugated NH-acids with heterocyclic N
2.1.2.1. Monocyclic N+H-acids
2.1.2.1.1. Three-membered rings
2.1.2.1.2. Four-membered rings
2.1.2.1.3. Five-membered rings
2.1.2.1.3.1. Pyrrolidines
2.1.2.1.3.1.1. Pyrrolidines with one
substituent
2.1.2.1.3.1.2. Pyrrolidines with two or
more substituents
2.1.2.1.3.2. 2-Pyrrolines
2.1.2.1.3.3. Pyrroles
2.1.2.1.3.4. 2,5-dihydroazoles
2.1.2.1.3.5. Pyrazoles
2.1.2.1.3.5.1. N-nonsubstituted
pyrazoles
2.1.2.1.3.5.2. N-substituted pyrazoles
2.1.2.1.3.5.2.1. Pyrazoles substituted
at N-1 by methyl
2.1.2.1.3.5.2.2. Pyrazoles substituted
at N-1 by ethyl
2.1.2.1.3.5.2.3. Pyrazoles substituted
at N-1 by various groups
2.1.2.1.3.6. 2-imidazolines
2.1.2.1.3.7. Imidazoles
2.1.2.1.3.7.1. N-nonsubstituted
imidazoles
2.1.2.1.3.7.1.1. Imidazoles with one substituent
2.1.2.1.3.7.1.2. Imidazoles with two or more substituents
2.1.2.1.3.7.1.3.
Imidazoles substituted by phenyl
2.1.2.1.3.7.2. N-substituted imidazoles
2.1.2.1.3.7.2.1. Imidazoles substituted at N-1 by methyl
2.1.2.1.3.7.2.2. Imidazoles substituted at N-1 by various groups
2.1.2.1.3.8. Triazoles
2.1.2.1.3.9. Tetrazoles
2.1.2.1.3.9.1. 1,2,3,4,-tetrazoles
2.1.2.1.3.9.2. 1,2,3,5,-tetrazoles
2.1.2.1.3.10. N-heterocycles with exocyclic double bonds
2.1.2.1.3.11. Benzo derivatives and other fused cycles
2.1.2.1.3.11.1. Fused cycles of imidazoles
2.1.2.1.3.11.1.1. Benzimidazoles
2.1.2.1.3.11.1.1.1. Benzimidazoles with one substituent
2.1.2.1.3.11.1.1.2. Benzimidazoles with two or more
substituents
2.1.2.1.3.11.1.1.3. Benzimidazoles substituted by cycle bridged
cycle
2.1.2.1.3.11.1.2. Other fused cycles of imidazoles
2.1.2.1.3.11.2. Fused cycles of other five-membered rings
2.1.2.1.4. Six-membered rings
2.1.2.1.4.1. Piperidines
2.1.2.1.4.1.1. Piperidines with one
substituent
2.1.2.1.4.1.2. Piperidines with two
substituents
2.1.2.1.4.1.3. Piperidines with three
substituents
2.1.2.1.4.1.4. Piperidines with four
substituents
2.1.2.1.4.1.5. Piperidines with five
substituents
2.1.2.1.4.1.6. Piperidines with
exocyclic double bond
2.1.2.1.4.1.7. Fused cycle of
piperidine
2.1.2.1.4.1.7.1. Decahydroquinolines
2.1.2.1.4.1.7.2. Benzofused piperidines
2.1.2.1.4.1.7.3. Othes fused cycles of piperidine
2.1.2.1.4.2. 2-pipereidines
2.1.2.1.4.3. 3-pipereidines
2.1.2.1.4.4. Piperazines
2.1.2.1.4.4.1. Piperazines
2.1.2.1.4.4.2. Benzofused piperazines
2.1.2.1.4.5. Pyridines
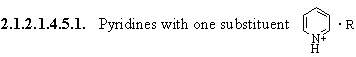
2.1.2.1.4.5.1.1. R = H
2.1.2.1.4.5.1.2. R = alkyl,
-CH=CH2
2.1.2.1.4.5.1.3. R =
functional group
2.1.2.1.4.5.1.3.1. R = halogen
2.1.2.1.4.5.1.3.2. R = -OX
2.1.2.1.4.5.1.3.3. R = -COX
2.1.2.1.4.5.1.3.4. R = -CSX
2.1.2.1.4.5.1.3.5. R = -NX2; N+X3
2.1.2.1.4.5.1.3.6. R = -NXCOX
2.1.2.1.4.5.1.3.7. R = -NHCSX
2.1.2.1.4.5.1.3.8. R = -CX=NX
2.1.2.1.4.5.1.3.9. R = -CN
2.1.2.1.4.5.1.3.10. R = -NO2
2.1.2.1.4.5.1.3.11. R = -SX
2.1.2.1.4.5.1.3.12. R = -S(O)X
2.1.2.1.4.5.1.3.13. R = -SiX3; -GeX3; -SnX3
2.1.2.1.4.5.1.3.14. R = -P(O)X2
2.1.2.1.4.5.1.4. R = phenyl
2.1.2.1.4.5.1.5. R = alkyl
substituted by functional group
2.1.2.1.4.5.1.5.1. R = alkyl substituted by halogen
2.1.2.1.4.5.1.5.2. R = alkyl substituted by OH-group
2.1.2.1.4.5.1.5.3. R = alkyl substituted by COX-group
2.1.2.1.4.5.1.5.4. R = alkyl substituted by NX2 or N+X3-group
2.1.2.1.4.5.1.5.5. R = alkyl substituted by CN or SCN-group
2.1.2.1.4.5.1.5.6. R = alkyl substituted by NO2-group
2.1.2.1.4.5.1.5.7. R = alkyl substituted by SX-group
2.1.2.1.4.5.1.5.8. R = alkyl substituted by SO2X-group
2.1.2.1.4.5.1.5.9. R = alkyl substituted by P(O)X-group
2.1.2.1.4.5.1.5.10. R = alkyl substituted by OP(O)(O-)OH-group
2.1.2.1.4.5.1.5.11. R = alkyl substituted by SiX3-group
2.1.2.1.4.5.1.5.12. R = alkyl substituted by -OOC(cycle)
2.1.2.1.4.5.1.5.13. R = alkyl substituted by phenyl
2.1.2.1.4.5.1.5.14. R = alkyl substituted by phenyl and
functional group
2.1.2.1.4.5.1.6. R = alkyl substituted by functional group
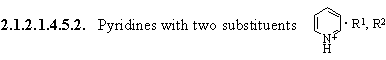
2.1.2.1.4.5.2.1. R1, R2 = alkyl
2.1.2.1.4.5.2.2. R1 =
Me, R2 = functional group
2.1.2.1.4.5.2.3. R1 =
alkyl, R2 = functional group
2.1.2.1.4.5.2.4. R1 =
halogen, R2 = functional group
2.1.2.1.4.5.2.5. R1 =
OH(O-), R2 = functional group
2.1.2.1.4.5.2.6. R1 =
O(alkyl), R2 = functional group
2.1.2.1.4.5.2.7. R1 =
-COOH, R2 = functional group
2.1.2.1.4.5.2.8. R1 =
NX2, R2 = functional group
2.1.2.1.4.5.2.9. R1, R2 = various groups
2.1.2.1.4.5.3. Pyridines with three substituents
2.1.2.1.4.5.4. Pyridines with four substituents
2.1.2.1.4.5.5. Pyridines with five substituents
2.1.2.1.4.5.6. Pyridines substituted by bridged cycles
2.1.2.1.4.5.7.
Pyridines with exocyclic double bond
2.1.2.1.4.5.8.
Polycyclic systems with one N-heteroatom
2.1.2.1.4.5.8.1. Benzofused pyridines
2.1.2.1.4.5.8.1.1.
Quinolines
2.1.2.1.4.5.8.1.1.1. Quinolines with one substituent
2.1.2.1.4.5.8.1.1.2. Quinolines with two substituents
2.1.2.1.4.5.8.1.1.3. Quinolines with three substituents
2.1.2.1.4.5.8.1.1.4. Quinolines with four substituents
2.1.2.1.4.5.8.1.1.5. Quinolines, substituted by bridged cycles
2.1.2.1.4.5.8.1.1.6. Quinolines with exocyclic double bonds
2.1.2.1.4.5.8.1.2. Isoquinolines
2.1.2.1.4.5.8.1.3. 6,7-benzquinolines
2.1.2.1.4.5.8.1.4. Acridines
2.1.2.1.4.5.8.1.5. 1-azaphenantrenes
2.1.2.1.4.5.8.1.6. 2-azaphenantrenes
2.1.2.1.4.5.8.1.7. 3-azaphenantrenes
2.1.2.1.4.5.8.1.8. 4-azaphenantrenes
2.1.2.1.4.5.8.1.9. Phenanthridines
2.1.2.1.4.5.8.1.10. 1,2-benzacridines
2.1.2.1.4.5.8.1.11. 2,3-benzacridines
2.1.2.1.4.5.8.1.12. 3,4-benzacridines
2.1.2.1.4.5.8.1.13. Other benzofused pyridines
2.1.2.1.4.5.8.2. Other polycyclic systems with one
N-heteroatom
(fused cycles of
pyridines)
2.1.2.1.4.6. Pyridazines
2.1.2.1.4.6.1. Pyridazines with one substituent
2.1.2.1.4.6.2. Pyridazines with two or more substituents
2.1.2.1.4.6.3. Pyridazines with exocyclic double bond
2.1.2.1.4.6.4. Fused cycles of pyridazines
2.1.2.1.4.7. Pyrimidines
2.1.2.1.4.7.1. Pyrimidines with one substituent
2.1.2.1.4.7.2. Pyrimidines with two substituents
2.1.2.1.4.7.3. Pyrimidines with three substituents
2.1.2.1.4.7.4. Pyrimidines with four substituents
2.1.2.1.4.7.5.
Hydroxypyrimidines
2.1.2.1.4.7.6.
Mercaptopyrimidines
2.1.2.1.4.7.7. Hydroxymercaptopyrimidines
2.1.2.1.4.7.8. Pyrimidines
with exocyclic double bond
2.1.2.1.4.7.8.1. Pyrimidines with exocyclic =O-group
2.1.2.1.4.7.8.2. Pyrimidines with exocyclic =S-group
2.1.2.1.4.7.8.3. Pyrimidines with exocyclic =NH-group
2.1.2.1.4.7.8.4. Pyrimidines with various exocyclic double
bond
2.1.2.1.4.7.9. Fused cycles of pyrimidine
2.1.2.1.4.7.9.1. Quinazolines
2.1.2.1.4.7.9.2. Hydrated quinazolines
2.1.2.1.4.7.9.3. Other fused cycles of pyrimidine
2.1.2.1.4.7.9.4. Fused cycles of pyrimidine with exocyclic double bond
2.1.2.1.4.8. Pyrazines
2.1.2.1.4.8.1. Pyrazines
2.1.2.1.4.8.2. Benzofused pyrazines
2.1.2.1.4.8.2.1. Quinoxalines
2.1.2.1.4.8.2.2. Phenazines
2.1.2.1.4.9. 1,2,4-Triazines
2.1.2.1.4.10. 1,3,5-Triazines
2.1.2.1.4.11. 1,2,4,5-Triazines
2.1.2.1.5. Seven-membered rings
2.1.2.1.5.1. Seven-membered rings
2.1.2.1.5.2. Benzo derivatives of seven-membered rings
2.1.2.1.6. Eight-membered rings
2.1.2.2.
Polycyclic N+H-acids (Fused N-heterocycles)
2.1.2.2.1. Bicyclic N+H-acids
2.1.2.2.1.1. Bicyclic systems contained two nitrogens
2.1.2.2.1.1.1. Bicyclic systems with two nitrogens contained six- and
five- or seven-membered rings
2.1.2.2.1.1.2. Bicyclic systems with two nitrogens contained
two six-membered rings
2.1.2.2.1.2. Bicyclic systems
contained three nitrogens
2.1.2.2.1.2.1. Bicyclic systems with three nitrogens contained five-
and six-membered rings
2.1.2.2.1.2.2. Bicyclic systems with three nitrogens contained
two six-membered rings
2.1.2.2.1.3. Bicyclic systems contained four
nitrogens
2.1.2.2.1.3.1. Bicyclic systems with four nitrogens contained five- and
six-membered rings
2.1.2.2.1.3.1.1. Pyrazolopyrimidines
2.1.2.2.1.3.1.2. 1-H-imidazopyridazines
2.1.2.2.1.3.1.3. Purines
2.1.2.2.1.3.1.3.1. Purines and its tautomeric derivatives
2.1.2.2.1.3.1.3.2. Purines with exocyclic double bond
2.1.2.2.1.3.1.4. 1H-imidazo[4,5-b]pyrazines
2.1.2.2.1.3.1.5. Imidazo[1,2-b]triazines
2.1.2.2.1.3.2. Bicyclic systems with
four nitrogens contained two
six-membered
rings
2.1.2.2.1.3.2.1. Pteridines
2.1.2.2.1.3.2.2. Hydropteridines
2.1.2.2.1.3.2.3. Pteridines with exocyclic double bond
2.1.2.2.1.3.2.4. Other bicyclic systems with four nitrogens
2.1.2.2.1.4. Bicyclic systems contained five nitrogens
2.1.2.2.2. Tricyclic N+H-acids
2.1.2.2.2.1. Tricyclic systems contained two nitrogens
2.1.2.2.2.1.1. Phenanthrolines
2.1.2.2.2.1.2. Other tricyclic systems with two
nitrogens
2.1.2.2.2.2. Tricyclic systems contained three
nitrogens
2.1.2.2.2.3. Tricyclic systems contained four
or five nitrogens
2.1.2.2.3. Four- and fivecyclic N+H-acids
2.1.3. Conjugated NH-acids with heterocyclic N and O
2.1.3.1. Monocyclic N+H-acids with heterocyclic N and O
2.1.3.1.1. Isoxazoles
2.1.3.1.2. 2,3-dihydroisoxazoles
2.1.3.1.3. 4,5-dihydroisoxazoles
2.1.3.1.4. Oxazoles
2.1.3.1.5. 1,2,5-oxadiazoles
2.1.3.1.6. 1,3,4-oxadiazoles
2.1.3.1.7. 6H-1,2-oxazines
2.1.3.1.8. Morpholines
2.1.3.2.
Polycyclic N+H-acids with heterocyclic
N and O
2.1.3.2.1. Anthranils (2,1-benzisoxales)
2.1.3.2.2. Benzoxazoles
2.1.3.2.3. Other polycyclic N+H-acids
2.1.4. Conjugated NH-acids with
heterocyclic N and S
2.1.4.1.
Monocyclic N+H-acids with heterocyclic
N and S
2.1.4.1.1. Thiazolidines
2.1.4.1.2. Thiazolines
2.1.4.1.3. Isothiazoles
2.1.4.1.4. Thiazoles
2.1.4.1.4.1. Thiazoles with one substituent
2.1.4.1.4.2. Thiazoles with two substituents
2.1.4.1.4.3. Thiazoles with three substituents
2.1.4.1.4.4. Thiazoles with exocyclic double bond
2.1.4.1.6. Thiomorphines
2.2. Nonconjugated NH-acids
2.2.1. Nonconjugated
NH-acids with noncyclic N
2.2.1.1. R2NH (R = H, alkyl)
2.2.1.2. R1CONHR2
2.2.1.2.1. RCONH2
2.2.1.2.2. R1CONHR2
(R2 = alkyl)
2.2.1.2.3. R1CONHOR2
2.2.1.2.4. R1CONHSR2
2.2.1.2.5. R1CONHSX=NSO2R2
2.2.1.2.6. R1CONHOC(O)R2
2.2.1.2.7. R1CONHCOR2
2.2.1.2.8. RCONHCONR1R2
2.2.1.2.9. RCONHNH2
2.2.1.2.10. R1CONHNHR2
2.2.1.2.11. RCONHNO2
2.2.1.2.12. RCONHCN
2.2.1.2.13.
RCONH(N=Heterocycle)
2.2.1.2.14. RCONHPh
2.2.1.2.15. PhCONHPh
2.2.1.2.16. R1NHCONHR2
2.2.1.2.17. R1NHCOOR2
2.2.1.2.18. R1NHCOON=CHR2
2.2.1.2.19. R1COCHXCONHR2
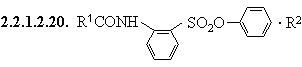
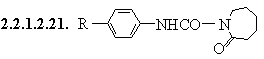
2.2.1.3. R1CSNHR2
2.2.1.3.1. R1CSNHR2
2.2.1.3.2. RCSNHPh
2.2.1.3.3. PhCSNHR
2.2.1.3.4. PhCSNHPh
2.2.1.3.5. RCSNHCH2Ph
2.2.1.3.6. PhCSNHCSNR1R2
2.2.1.3.7. R1NHCSNHR2
2.2.1.3.8. R1CONR2CSNHR3
2.2.1.3.9. R1OC(S)NHR2
2.2.1.3.10. R1SC(S)NHR2
2.2.1.3.11. (N-heterocycle)CSNHR
2.2.1.3.12. Various
thioamides
2.2.1.4. R1CONHCSR2
2.2.1.4.1. R1CONHCSNR2R3
2.2.1.4.2. R1CONHCSOR2
2.2.1.4.3. R1CONHCSNHCOR2
2.2.1.4.4. R1CONHCSR2
2.2.1.5. R1CONHCSeR2
2.2.1.6. R1SO2NHR2
2.2.1.6.1. R1SO2NH2
2.2.1.6.2. R1SO2NH(subst. phenyl)
2.2.1.6.3. (subst. phenyl)SO2NHR
2.2.1.6.4. (subst. phenyl)SO2NHPh
2.2.1.6.5. PhSO2NH(subst. phenyl)
2.2.1.6.6. (subst. phenyl)SO2NH(subst. phenyl)
2.2.1.6.7. (subst. phenyl)SO2NHS(subst. phenyl)
2.2.1.6.8. R2NSO2NH(subst. phenyl)
2.2.1.6.9. RSO2NH(heterocycle)
2.2.1.6.10. (Heterocycle)SO2NHR
2.2.1.6.11. R1SO2NHNHR2
2.2.1.6.12. R1NHSO2NHR2
2.2.1.6.13. R1NMeSO2NHR2
2.2.1.6.14. R1SO2NHSO2R2
2.2.1.6.15. R1SO2NHNHSO2R2
2.2.1.6.16. R1SO2NHR2
2.2.1.6.17. R1SO2NHCOR2
2.2.1.6.18. R1SO2NHCOCONHR2
![]()
2.2.1.6.20. R1SO2NHCOCONHCH2CH2R2
2.2.1.6.21. R1SO2NHCOCONHNHR2
2.2.1.6.22. R1SO2NHNHCOR2
2.2.1.6.23. R1SO2NHNHCOCH2CH2CONHR2
2.2.1.6.24. R1SO2NHNHCOCONHR2
2.2.1.6.25. R1SO2NHNHCOCONHSO2R2
2.2.1.6.26. R1SO2NHNHCOCONHNHSO2R2
2.2.1.6.27. R1SO2NHCSR2
2.2.1.6.28. R1SO2NHPSR2
2.2.1.6.29. R1SO2NHSP(S)(OEt)2
2.2.1.7. R1NHP(O)R2R3
2.2.1.8. RNHNO2
2.2.1.9. RNH(phenyl)
2.2.1.9.1. PhNH2
2.2.1.9.2. PhNHR
2.2.1.9.3. PhNHPh
2.2.1.9.4. PhNH(carbocycle)
2.2.1.10. RNH(naphthyl)
2.2.1.11. RNH(heterocycle)
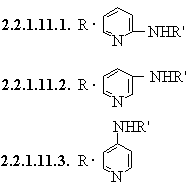
2.2.1.11.4.
RNH(N-heteromonocycle)
2.2.1.11.5. RNH(N-heterobicycle)
2.2.1.12. RNHCX=CHCSR’
2.2.1.13. RNHCX=NR’
2.2.1.14. RNHN=CR1R2
2.2.1.15. R1NHN=CXP(O)R2
2.2.1.16. R1NHN=CXN=NR2
2.2.1.17. RNHN=(-cycle-)
2.2.1.18. (subst. phenyl)NHN=N(subst. phenyl)
2.2.2. Nonconjugated NH-acids with heterocyclic N
2.2.2.1.
Monocyclic NH-acids
2.2.2.1.1. Five-membered
rings
2.2.2.1.1.1. Pyrroles
2.2.2.1.1.2. Pyrazoles
2.2.2.1.1.3. Imidazoles
2.2.2.1.1.4. 1,2,3-Triazoles
2.2.2.1.1.5. 1,2,4-Triazoles
2.2.2.1.1.6. 1,2,3,4-Tetrazoles
2.2.2.1.1.7. 1,2,3,5-Tetrazoles
2.2.2.1.1.8. Benzo derivatives of five-membered rings
2.2.2.1.1.8.1. Indoles
2.2.2.1.1.8.2. 1H- Indazoles
2.2.2.1.1.8.3. Benzimidazoles
2.2.2.1.1.8.4. Benzotriazoles
2.2.2.1.1.9. Other fused cycles of five-membered rings
2.2.2.1.2. Six-membered rings
2.2.2.1.2.1. Nonfused cycles
2.2.2.1.2.2. Fused cycles
2.2.2.2.
Polycyclic NH-acids
2.2.2.2.1. Bicyclic systems contained two nitrogens
2.2.2.2.2. Bicyclic systems contained three nitrogens
2.2.2.2.3. Bicyclic systems contained four nitrogens
2.2.2.2.4. Bicyclic systems contained five nitrogens
2.2.2.2.5. Tricyclic systems
2.2.3. Nonconjugated
NH-acids with heterocyclic N and O
2.2.4. Nonconjugated NH-acids with heterocyclic N and S