A multicomponent QSPR approach to describe and predict the distribution of organic solutes in gas-ionic liquid environments using machine learning
Uko Maran
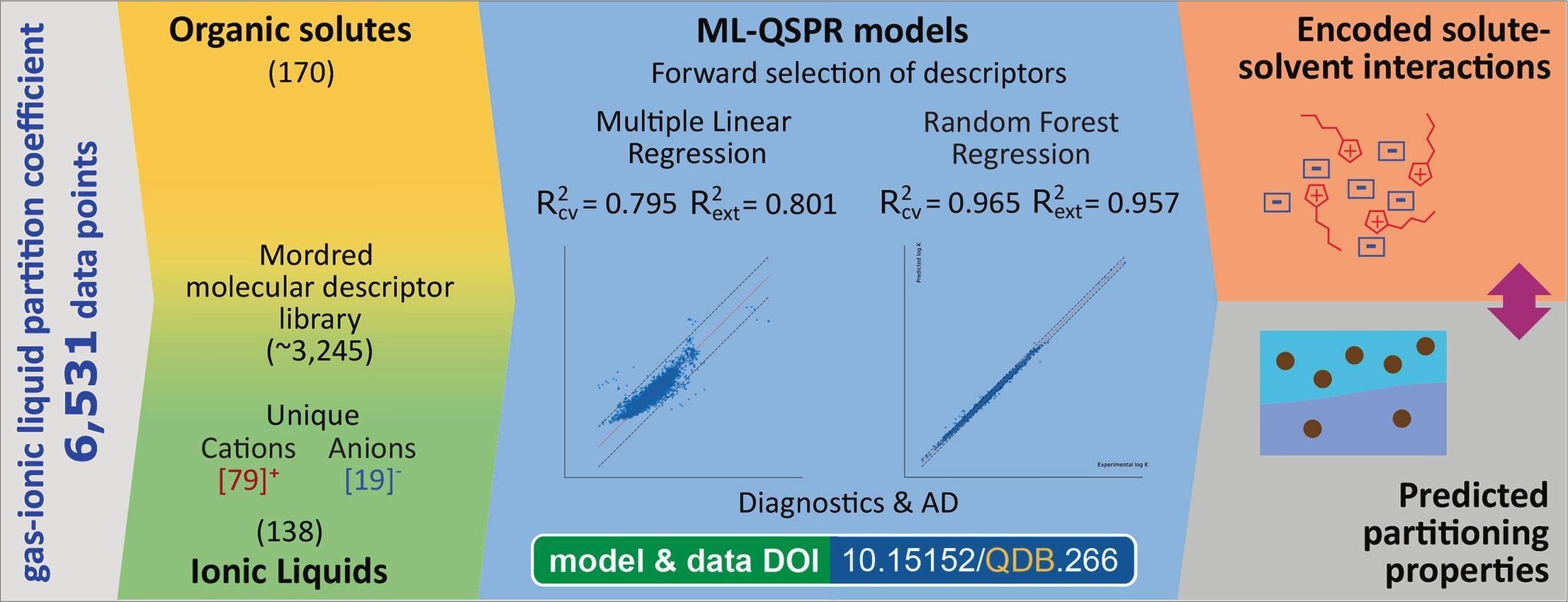
Ionic liquids are used as green solvents, which make accurate predictions of gas–ionic liquid partition coefficients (log K) of organic solutes important from the perspective of various industrial applications. An ionic liquid with an interacting organic solute is a multicomponent system that is usually modelled based on the structural properties of one component. The integration of structural descriptors of all three components – solute, cation, and anion - into a single model has not yet been accomplished. In order to achieve this, a machine learning approach was applied to a large, collected data set consisting of 6531 experimental log K values, including data series for 170 solutes and 138 ionic liquids. Multiple Linear Regression (MLR) and Random Forest Regression (RF) were compared, both using stepwise selection of descriptors. The model for MLR achieved a cross-validated coefficient of determination R2cv=0.795, cross-validated root mean square error RMSEcv=0.469 and an external validation coefficient of determination R2=0.801 (RMSE=0.470), while the model for RF demonstrated a significant increase in performance with R2cv=0.965 (RMSEcv=0.193) and external validation R2=0.957 (RMSE=0.220). The descriptors in the models showed that the description and prediction of log K are significantly improved when the structural properties of the solute, cation, and anion are simultaneously included in the model. A comparison of the two models showed a significant increase in the presence of molecular descriptors of ionic components in the non-linear model. The descriptors highlighted the roles of dispersion forces, dipolar interactions, and hydrogen bonding in solute–ionic liquid partitioning. The study provides thoroughly-analysed predictive models for estimating gas–ionic liquid partition coefficients of organic solutes and provides structure-level insights into solute–ionic-liquid interactions, facilitating the rational design of ionic liquids and expanding the range of solutes for various applications.
Article: https://doi.org/10.1016/j.molliq.2025.128184
FAIR data and models: https://doi.org/10.15152/QDB.266
